Reading Passages on Changing States of Matter
In your daily activities, y'all volition observe if ice is heated it changes into the water if water is heated it changes into steam if steam is cooled it changes into the water if h2o is cooled information technology changes into ice. This is a daily life instance where we all see the application of the change in the state of the matter, but exercise you ever retrieve that How is it possible? How this transformation takes place? And on what it all depends? Don't worry you will become answers to all your questions in this commodity.
Change of State of Matter
Solid-state, liquid land, and gaseous state are 3 states of matter, and any physical alter in their country is called a modify of state of matter. These changes are reversible in nature means they tin can attain any state again and once again. This reversible property of the three states depends upon different parameters and conditions which will be discussed below.
A physical change in a matter is referred to as a change of country of matter. They are reversible modifications that do non crave any changes in the thing'southward chemical limerick. Melting, freezing, sublimation, degradation, condensation, and vaporisation are examples of common state transitions.
We can understand the meaning of a alter of states of affair in ane more manner i.e. when a solid is heated it changes into liquid, and when a liquid is heated it changes into a gas, and when a gas is cooled it changes to a liquid when a liquid is cooled it changes to solid. And, we can interchange these states by:
- Changing the temperature
- Irresolute the pressure
Why do States of Thing Change?
The modify in state occurs due to the following factors:
- The change in intermolecular space and strength of attraction,
- The modify in temperature,
- The change in pressure and
- The alter in kinetic free energy of the particle.
Permit's discuss each bespeak in more depth equally
1. Past Changing the Temperature: The temperature result on heating a thing depends upon the nature of the affair and the conditions required in bringing the modify. So, let's hash out all the vi interchanges betwixt these states now.
- Solid to Liquid change- This process is known as Melting. The process in which a solid substance changes into a liquid on heating is chosen melting. On increasing the temperature of the solid the kinetic energy of the particle increase which overcomes the force of attraction betwixt the particles thereby solid melts and is converted into liquid.
- Liquid to Gas alter- This process is known as Humid or Vapourisation. The process in which a liquid changes into gas apace on heating is chosen boiling. The temperature at which a liquid boils and changes rapidly into the gas at atmospheric pressure is called the boiling point of the liquid.
- Gas to Liquid change- This process is known as Condensation. The process of changing gas into liquid past cooling is called condensation. Condensation is the reverse of boiling.
- Liquid to Solid change- This process is known equally Freezing. The process of transformation of liquid into a solid by cooling is called freezing. Freezing means solidification. Information technology is the reverse of the melting process.
- Solid to Gas modify- This process is known as Sublimation. The change of solid straight into vapor on heating without passing through the intervening liquid state is called sublimation. The common substances which undergo sublimation are ammonium chloride, iodine, camphor, naphthalene, and anthracene. east.g. Solid carbon dioxide(or dry ice) sublimes to class carbon dioxide gas. Naphthalene balls disappear with time without leaving behind any residue.
- Gas to Solid alter- This process is known as Deposition or Desublimation. It is a thermodynamic procedure in which gas changes into a solid directly without inbound into the liquid phase.
ii. By Irresolute the Pressure level: The physical state of thing can also be changed by irresolute the force per unit area. By applying loftier pressure the particles of a gas can exist brought close together ways gases can be liquified hands past applying pressure level and reducing temperature. When pressure level is applied particles come up together thus the force of attraction increases and intermolecular space decreases. Hence, gas liquefies. When force per unit area around the solid carbon dioxide is reduced its temperature increases and it direct changes into carbon dioxide gas.
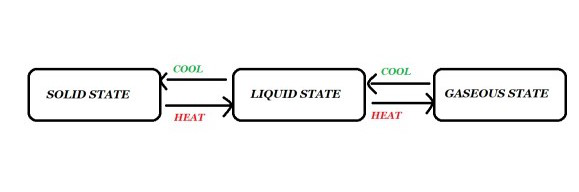
Interconversion of Three States of Matter
Usa of matter are interconvertible. The country of thing can be changed by changing the temperature or pressure. The transition of ane state to another is referred to as the interconversion of matter. Information technology is a process in which affair transitions from 1 country to another and and then returns to its original state with no change in its chemical makeup. Heating may transform solids into liquids. These changes are shown in the figure given below.

Interconversion of 3 States of Matter
Of import Definition related to Alter of State of Thing
- Melting Signal: The temperature at which a solid melts to grade a liquid at atmospheric pressure is called its melting betoken. During melting the temperature of ice does not rising even though rut is being supplied continuously due to latent estrus of fusion this latent rut is used to overcome the forcefulness of attraction between the particles of ice.
- Boiling betoken: The temperature at which a liquid boils to form vapors at atmospheric pressure is called its humid point. The humid bespeak of water is 373 grand.
- Latent Heat: The oestrus energy which has to be supplied to modify the state of a substance is chosen latent heat. Information technology is called latent heat considering it becomes/gets hidden in the substance changing state and does non prove its presence by raising the temperature. The latent oestrus which we supply is used up in overcoming the forces of attraction between the particles of a substance during the alter of state. Latent estrus is of two types: The latent heat of fusion and the latent rut of vaporization.
- Latent Heat of Fusion (solid to liquid change): The latent heat of fusion( or melting )of a solid to liquid is the quantity of heat in joules required to convert 1 kilogram of the solid (at its melting betoken) to liquid without any temperature change.
- Latent Heat of Vaporization (liquid to gas change): During humid, the temperature of the h2o does not right fifty-fifty do heat is being supplied continuously as this heat of vaporization is used to overcome the force of attraction between h2o particles.
- Freezing indicate: Freezing is the transformation of liquid water into solid ice. The freezing point is the temperature at which it happens.
- Boiling point: The process through which a liquid boils and transforms into a gas is known equally vaporization. The boiling point of a liquid is the temperature at which it begins to eddy.
- Condensation: The mirror is decumbent to fog upward when you have a hot shower in a closed bathroom. You lot may exist wondering why this occurs. When hot water evaporates from the shower, it cools and loses energy when it comes into touch on with colder surfaces such every bit the mirror. Cooler water particles lack the free energy to overcome the forces of attraction between them. They combine to create droplets of liquid h2o. Condensation is the process through which gas transforms into a liquid.
- Vapourization: If the water is sufficiently heated, it begins to boil. Water vapor bubbles develop in the boiling water. This occurs when liquid h2o particles gather enough energy to totally overcome the force of attraction between them and transition to the gaseous form. The bubbles rising through the water and leave as steam from the bucket. The process through which a liquid boils and transforms into a gas is known equally vaporization. The boiling point of a liquid is the temperature at which it begins to boil.
- Sublimation: Sublimation is the process through which solids immediately transform into gases. When solids absorb enough energy to totally overcome the forces of allure betwixt them, this happens. Dry ice is an example of solids that undergo sublimation.
- Evaporation: The modify of a liquid into vapor at whatsoever temperature beneath its boiling point is called evaporation. It is a surface phenomenon in which particles from the surface gain enough energy to overcome the forces of attraction. This operation causes cooling because when a liquid evaporates the particles of the liquid blot estrus from the surroundings and its surrounding get cool. Particles on the surface of a liquid have higher kinetic free energy than others, then they intermission the forces of attraction between the particle and escape from the surface of the liquid in the form of vapors.
Sample Bug
Problem 1: Why practise we wear cotton clothes in summer?
Solution:
We should wear cotton fiber clothes in summer to stay cool and comfortable, as cotton wool is good absorber of water, so it absorbs the sweat from the body and exposes it to air for evaporation of sweat. Thus, cools our body.
Problem ii: Why solid CO2 is known as dry out ice?
Solution:
Solid COii get converted direct to gaseous state on subtract of pressure to 1 atm without coming into liquid state that's why it is also known as Dry water ice. Too, because of this it is placed or stored under loftier pressure.
Problem iii: Why does evaporation always cause cooling?
Solution:
The cooling caused by evaporation is based on the fact that when liquid evaporates it takes latent rut of vaporization from surrounding, which on losing oestrus get cooled.
Trouble 4: Why do nosotros feel absurd when nosotros utilize acetone to our palms?
Solution:
When we put Acetone on our hand, it gets vaporated by taking heat from our hand and our manus feels cool.
Problem v: Why gases can be compressed hands just not liquids?
Solution:
This is considering particles of gas have huge intermolecular space between them thus have tendency to shrink but liquids have already less intermolecular space therefore can't be compress more.
Problem 6: How humidity affects the process of evaporation?
Solution:
When humidity of the air is depression, evaporation rate is increased. Thus more humidity denotes less evaporation.
johnsonprepireare.blogspot.com
Source: https://www.geeksforgeeks.org/changing-states-of-matter/
0 Response to "Reading Passages on Changing States of Matter"
Post a Comment